Your support is invaluable
The Situation
PNH is a serious, very rare disease which affects people in their early adult life (average age at diagnosis mid thirties). The disease is characterised by excessive haemolysis, or the destruction of red blood cells, which can lead to a range of serious health problems such as thromboses (formation of blood clots), pulmonary hypertension and acute and or chronic renal failure.
22 diagnosed cases of PNH are recorded on the New Zealand PNH patient registry. According to the Haematology Society of Australia and New Zealand (HSANZ) approximately 8-10 patients are considered to have severe PNH.
Supportive care is currently offered to PNH patients in New Zealand. This approach aims to mitigate the symptoms of the disease but does not address the fundamental disease mechanism, haemolysis, which is responsible for the most serious and life-threatening complications of the condition. Bone Marrow Transplantation is the only curative therapy for PNH but is associated with high morbidity and mortality and is generally only considered as a last resort.
Soliris is a treatment for PNH which is funded in more than 40 countries internationally; Australia, Argentina, Brazil, Canada, Denmark, England, France, Germany, Ireland, Israel, Italy, Japan, Korea, Taiwan, Wales, to name a few.
On December 12 the funding application for Soliris in NZ was declined. PHARMAC, the government's medicines funding agency, have cited cost as the barrier while also noting: "Treatment with eculizumab would potentially result in reduced transfusion requirements, reduced haemolysis, improved haemoglobin levels, improved fatigue and quality of life, reduced thrombosis rates and prolonged survival." Source: PHARMAC's Memorandum of Board Meeting (2 December 2013)
22 diagnosed cases of PNH are recorded on the New Zealand PNH patient registry. According to the Haematology Society of Australia and New Zealand (HSANZ) approximately 8-10 patients are considered to have severe PNH.
Supportive care is currently offered to PNH patients in New Zealand. This approach aims to mitigate the symptoms of the disease but does not address the fundamental disease mechanism, haemolysis, which is responsible for the most serious and life-threatening complications of the condition. Bone Marrow Transplantation is the only curative therapy for PNH but is associated with high morbidity and mortality and is generally only considered as a last resort.
Soliris is a treatment for PNH which is funded in more than 40 countries internationally; Australia, Argentina, Brazil, Canada, Denmark, England, France, Germany, Ireland, Israel, Italy, Japan, Korea, Taiwan, Wales, to name a few.
On December 12 the funding application for Soliris in NZ was declined. PHARMAC, the government's medicines funding agency, have cited cost as the barrier while also noting: "Treatment with eculizumab would potentially result in reduced transfusion requirements, reduced haemolysis, improved haemoglobin levels, improved fatigue and quality of life, reduced thrombosis rates and prolonged survival." Source: PHARMAC's Memorandum of Board Meeting (2 December 2013)
The human costThe real cost of PHARMAC's decision to decline funding for the Soliris treatment is the lost contributions of the New Zealanders affected by the PNH disease.
BackgroundAs highlighted in various reviews and reports over the past decade, New Zealand has an appalling record on the issue of access to orphan drugs. The argument from patients and supporters is simple, New Zealand's approach is out of step with the rest of the developed world. More explicitly, the wholesale application of a narrow pharmacoeconomic principal is inherently unethical, undermines the right to life and right to health of patients, and is resulting in unnecessary suffering and premature death.
In 2006 PHARMAC commissioned the public consultation "How should high cost medicines be funded?". The conclusion from this consultation arrived at a particularly glib justification for the status quo; "There are a number of ethical theories that can be applied to how high cost (or indeed any) medicines are assessed. How decisions are made will depend on the set of values used." No substantive changes resulted from this report, and patients living with rare disease continued to be denied access to treatment. In 2008, after signalling improved access to highly specialised medicines during the election, incoming Health Minister Tony Ryall commissioned a report to look into the issue. In 2010 the "Review of access to high cost, highly specialised medicines" was released. The conclusion from this report: "In theory, if access to high cost medicines were to be increased then, logically, the funds to pay for more high cost medicines must come from at least one of these three main sources: (1) increased government spending, (2) increased patient co-payments on prescriptions overall, and (3) reduced wastage (i.e. better value for money) in the medicines system and/or health system overall. We recommend that you pursue the third of these options". Following this report, patients living with rare disease continued to be denied access to treatment. In 2013 the "Ombudsman consideration of Pharmac decision not to approve funding for Myozyme" was released. One of the conclusions of this report was "that the decision criteria under the NPPA Policy ought to be clearly differentiated from those under the Pharmaceutical Schedule." This is an explicit message to PHARMAC; your one size fits all approach MUST be reviewed. To date, Pharmac has signalled no changes which would allow patients living with a rare disease access to life restoring and life saving therapies. Economic impact is ONE of many considerationsPNH patients in New Zealand have waited two years to be told; Yes, you have a "high clinical need". Yes, a treatment exists which meets this need. No, that treatment will not be funded.
Clearly, without a substantive change to the way in which PHARMAC considers orphan treatments, patients in New Zealand will continue to be denied access to these treatments and as a result, suffer unnecessarily and die prematurely. Is our claim to the right to health, to the right to life, exceptional? We do not think so. Are these claims being routinely dismissed in New Zealand through the indiscriminate application of a narrowly defined pharmacoeconomic principle. Clearly, yes. Do we claim to have all the answers? No. Funding decisions need to be weighed carefully and economic impact is ONE of the factors in making a good decision. However, when the equivalent of the combined salaries of four state sector bosses cannot be found to restore health to a group of ten patients, we feel there is a distortion in priorities. Before we accept that good decisions can be reached solely on the basis of a universally applied cost/ benefit analysis, it is worth pausing for a moment to consider where this may lead. Suffice to say, the rationale of a public health system is of equitable access to ALL. |
What do other countries say?Orphan drugs are the product of a complex, some may say flawed, economic environment. PNHSANZ accepts there is significant work to be done toward establishing a transparent value index for orphan drugs, particularly in respect of their high cost. However, in lieu of such an index, PNHSANZ have reviewed the publicly available evaluations and policy frameworks for supply of Soliris in Australia, England, Canada and Sweden.
In reviewing these policy documents, a qualitatively different focus, perhaps better described as a disposition, is immediately evident. The UK's National Institute for Health and Care Excellence (NICE) provides a useful summary; “Given the very small numbers of patients living with these very rare conditions a simple utilitarian approach, in which the greatest gain for the greatest number is valued highly, is unlikely to produce guidance which would recognize the particular circumstances of these very rare conditions. These circumstances include the vulnerability of very small patient groups with limited treatment options, the nature and extent of the evidence, and the challenge for manufacturers in making a reasonable return on their research and development investment because of the very small population treated.” Source: Highly Specialised Technologies programme: Interim process and methods (May 2013) Or from the Swedish assessment of Soliris (HTA-report 2012:43); "The fundamental ethical conflict stands between equal rights to medical treatment for all humans as opposed to risks for displacement effects due to very high costs. The exact patient population that will benefit from treatment is not well defined. On the other hand, to refrain from the treatment for purely economic reasons is an ethically problematic strategy." By contrast, from the Memorandum of Board Meeting (2 Dec 2013) the following statement can be found; "PHARMAC staff welcomed these submissions and took the opportunity to consider the alignment of PHARMAC’s processes with its human rights obligations and other legal requirements, including by seeking independent legal advice. PHARMAC staff acknowledge the fundamental importance of human rights, and note that the legal advice received indicates that under PHARMAC’s current approaches, these obligations are being met." In the above it sounds curiously as though "human rights" are a sub-set of the "legal requirements" category. For patients being denied access to a live-saving treatment, this is little consolation. |
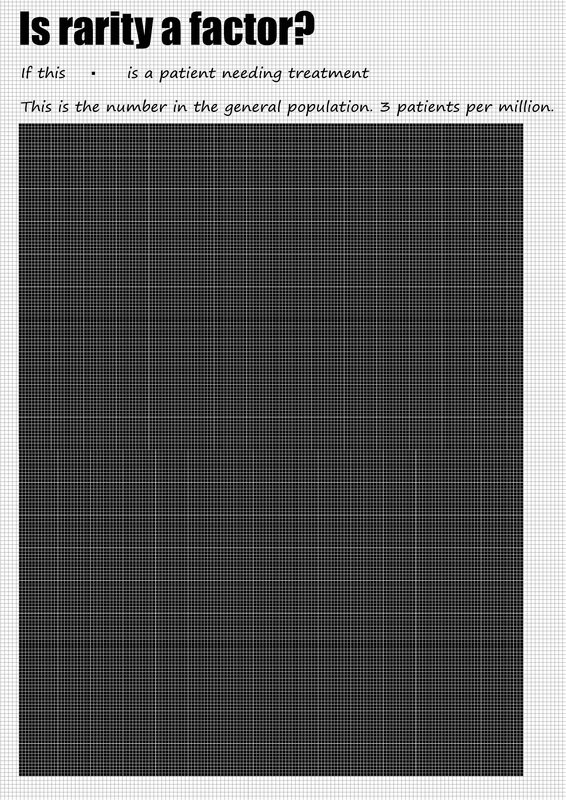
Patient numbersIn August 2012, PHARMAC's expert haematolgy subcommittee proposed access criteria to Soliris which would result in "approximately 3 patients per million population" and considered that "there is a high clinical need in this group of patients given the limited effective treatment alternatives." This would result in an upper limit of 13 patients in New Zealand.
The Swedish assessment of Soliris in PNH (HTA-report 2012:43) concludes that: "PNH is a rare disease. Reliable incidence and prevalence data are largely lacking. According to a retrospective study (Hill et al., 2007), the estimated incidence in Britain is 1.3/million/year and the prevalence 15.9/million." Incidence here is the relevant consideration, suggesting a target population in New Zealand of 6. PHARMAC have also based their estimate of patient numbers on the 2007 Hill et al. research, yet suggest the target patient population for treatment with Soliris in New Zealand would be "up to 20". Real world experience "A preliminary survey of New Zealand haematologists conducted by the Haematology Society of Australia and New Zealand (HSANZ) has highlighted that as at December 2013 there are approximately 8-10 known patients with severe PNH in New Zealand." Source: PHARMAC's Memorandum of Board Meeting (2 December 2013) "In Sweden, there are about 50 known patients with PNH (Database for rare diseases, Swedish National Board of Health and Welfare)" and "approximately 14 patients currently treated with eculizumab" (HTA-report 2012:43). With a total population of 9.5m, 1.5 patients per million are receiving the Soliris treatment in Sweden. PNHSANZ have consulted with our Australian colleagues and have been advised that there are currently 67 patients receiving the Soliris treatment, or just below 3 patients per million. Professor Peter Hillmen, Professor of Haematology at the University of Leeds and a senior figure in the National PNH Service (England) confirmed in a letter dated 14 May 2012; "In the UK we have been using eculizumab in PNH on the basis of clear guidelines and have 3 patients on therapy per million population. There is no reason that this would be different in New Zealand." What to conclude? In light of international evidence 8-13 patients is a fair assessment of the likely target population for treatment in New Zealand. Approximately half of what has been asserted by PHARMAC in publicly available materials from May 2013. In the interests of transparency, particularly given the high cost of Soliris, PNHSANZ feels the debate around access needs to be informed by accurate and reliable information. |
What is the cost of Soliris?
From the documents PHARMAC have released over the course of 2013, a range of per patient costs are detailed;
From PHARMAC decision on eculizumab (Soliris) funding (12 December 2013);
"Eculizumab could benefit up to 20 people, at a cost of approximately $10 million" or $500,000 pp
From PHARMAC decision on eculizumab (Soliris) funding (12 December 2013);
"Eculizumab is priced at about $670,000 per patient"
From Proposal to decline a funding application for eculizumab (21 May 2013);
"funding eculizumab for PNH in New Zealand would cost approximately $12,000,000 (20 patients) per year" or $600,000 pp
From PHARMAC's Memorandum of Board Meeting (2 December 2013);
"Net drug cost for 16 patients: $11M" or $687,500 pp
From PHARMAC decision on eculizumab (Soliris) funding (12 December 2013);
"Eculizumab could benefit up to 20 people, at a cost of approximately $10 million" or $500,000 pp
From PHARMAC decision on eculizumab (Soliris) funding (12 December 2013);
"Eculizumab is priced at about $670,000 per patient"
From Proposal to decline a funding application for eculizumab (21 May 2013);
"funding eculizumab for PNH in New Zealand would cost approximately $12,000,000 (20 patients) per year" or $600,000 pp
From PHARMAC's Memorandum of Board Meeting (2 December 2013);
"Net drug cost for 16 patients: $11M" or $687,500 pp
What would it cost to fund Soliris in New Zealand?
On the basis of advice from the Haematology Society of Australia and New Zealand's (8-10 patients with severe PNH) and considering international experience which confirms that a target population of 3 per million is appropriate (13 patients), a fair assumption of the total cost to treat PNH patients in New Zealand would be $4M increasing to $6.5M per year over the next 5 years. This represents less than 0.5% of the community medicines budget. These figures are calculated using the documented per patient cost of $500,000 as described above.
PNHSANZ fully support PHARMAC negotiating to achieve the best possible price for this treatment for New Zealand patients. However, PNHSANZ condemn the use of brinkmanship as a commercial negotiation tactic where patients lives are at stake.
PNHSANZ fully support PHARMAC negotiating to achieve the best possible price for this treatment for New Zealand patients. However, PNHSANZ condemn the use of brinkmanship as a commercial negotiation tactic where patients lives are at stake.
Negotiation
PNH patients in New Zealand are tired of being caught in the middle of a commercial negotiation between PHARMAC and Alexion Pharmaceuticals.
PNHSANZ understand that a revised commercial proposal was made by Alexion to PHARMAC in March 2013. We are not aware of the specific details of the proposal, other than that several funding models and discounting mechanisms were proposed. We have been advised that the proposal was followed by discussion in which further verbal proposals and counter-proposals were discussed. It is evident that negotiations have now stalled.
In their 12 December media release PHARMAC state that they "are hoping to get around the negotiating table with Alexion" in order that they "can get on with making this drug available for New Zealanders with PNH.”
PNHSANZ propose that if both parties are serious in their intention to enter into an agreement for the benefit of PNH patients in New Zealand, arbitration must be sought. We are in the process of writing an open letter to both parties to outline our concerns at the stalled negotiations and provide suggestions for recommencing dialogue.
Comparison with the UK
In the Memorandum of Board Meeting, PHARMAC have included the price for a 300mg vial of Soliris as detailed on the British National Formulary. This would equate to approximately $450,000 per patient.
PNHSANZ welcomes the use of international comparison as a means of achieving a fair price for Soliris. As such, it is deeply disappointing to read in the same document that; "even if those prices were obtained here, it would not alter the substance of our recommendation." In other words, even if the price offered was below international comparisons, PHARMAC would be unlikely to fund Soliris in New Zealand.
PNHSANZ understand that a revised commercial proposal was made by Alexion to PHARMAC in March 2013. We are not aware of the specific details of the proposal, other than that several funding models and discounting mechanisms were proposed. We have been advised that the proposal was followed by discussion in which further verbal proposals and counter-proposals were discussed. It is evident that negotiations have now stalled.
In their 12 December media release PHARMAC state that they "are hoping to get around the negotiating table with Alexion" in order that they "can get on with making this drug available for New Zealanders with PNH.”
PNHSANZ propose that if both parties are serious in their intention to enter into an agreement for the benefit of PNH patients in New Zealand, arbitration must be sought. We are in the process of writing an open letter to both parties to outline our concerns at the stalled negotiations and provide suggestions for recommencing dialogue.
Comparison with the UK
In the Memorandum of Board Meeting, PHARMAC have included the price for a 300mg vial of Soliris as detailed on the British National Formulary. This would equate to approximately $450,000 per patient.
PNHSANZ welcomes the use of international comparison as a means of achieving a fair price for Soliris. As such, it is deeply disappointing to read in the same document that; "even if those prices were obtained here, it would not alter the substance of our recommendation." In other words, even if the price offered was below international comparisons, PHARMAC would be unlikely to fund Soliris in New Zealand.
The voice of patients
The PNHSANZ was created because patients recognised a need. We took advice from other patient advocacy organisations and were given unequivocal support for the establishment of a patient support network and for providing a means for the collective voicing of patient rights.
We asked Alexion Pharma for a $900 grant without any conditions to help us start out, which was provided. No other financial assistance has been asked for or received. PNHSANZ also engaged Viva communications in the first half of 2013 to help us conduct patient interviews and prepare and distribute media releases. Viva's bills were paid for by Alexion.
New Zealanders living with PNH, and patients living with rare disease more broadly, are struggling for recognition of basic human rights - a struggle which seems all too easily dismissed by a state agency with significant resources at it's disposal. PNH patients are caught between two very powerful entities engaged in a commercial negotiation - all we have to fight with is a plea to humanity.
We asked Alexion Pharma for a $900 grant without any conditions to help us start out, which was provided. No other financial assistance has been asked for or received. PNHSANZ also engaged Viva communications in the first half of 2013 to help us conduct patient interviews and prepare and distribute media releases. Viva's bills were paid for by Alexion.
New Zealanders living with PNH, and patients living with rare disease more broadly, are struggling for recognition of basic human rights - a struggle which seems all too easily dismissed by a state agency with significant resources at it's disposal. PNH patients are caught between two very powerful entities engaged in a commercial negotiation - all we have to fight with is a plea to humanity.