Eculizumab (Soliris)
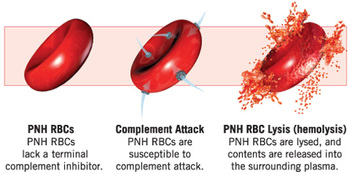 Complement activation without Soliris
Complement activation without Soliris
Eculizumab (brand name Soliris) has been approved for use in PNH by MedSafe but is not funded under public healthcare in New Zealand.
Eculizumab (pronounced E-cloo-zee-mab) is a monoclonal antibody which binds to red blood cells and interrupts the complement activation process, referred to as the complement cascade. By interrupting complement activation, defective red blood cells (PNH clones) are prevented from being destroyed in the bloodstream.
Eculizumab is administered intravenously every 14 days and is highly effective in treating patients with moderate to severe PNH. By stopping the destructive process of the PNH disease, eculizumab significantly reduces symptoms of PNH and in many cases haemoglobin levels are maintained without the need for blood transfusions. Eculizumab improves quality of life for patients and reduces the risk of life-threatening complications. Click here for international research into the efficacy of Eculizumab.
Side effects
Because Eculizumab interupts the complement cascade, patients under treatment have an increased risk of contracting a meningococcal infection. As a result patients must receive an effective meningococcal vaccine prior to commencing treatment with Eculizumab.
PLEASE NOTE: This information has been prepared as a guide for patients. It is not to be substituted for medical advice and accuracy is not guaranteed. Please consult with your medical practitioner for further information regarding your personal circumstances.
This information has been prepared by PNHSAA Inc., is copyright protected and is used with their permission.
Eculizumab (pronounced E-cloo-zee-mab) is a monoclonal antibody which binds to red blood cells and interrupts the complement activation process, referred to as the complement cascade. By interrupting complement activation, defective red blood cells (PNH clones) are prevented from being destroyed in the bloodstream.
Eculizumab is administered intravenously every 14 days and is highly effective in treating patients with moderate to severe PNH. By stopping the destructive process of the PNH disease, eculizumab significantly reduces symptoms of PNH and in many cases haemoglobin levels are maintained without the need for blood transfusions. Eculizumab improves quality of life for patients and reduces the risk of life-threatening complications. Click here for international research into the efficacy of Eculizumab.
Side effects
Because Eculizumab interupts the complement cascade, patients under treatment have an increased risk of contracting a meningococcal infection. As a result patients must receive an effective meningococcal vaccine prior to commencing treatment with Eculizumab.
PLEASE NOTE: This information has been prepared as a guide for patients. It is not to be substituted for medical advice and accuracy is not guaranteed. Please consult with your medical practitioner for further information regarding your personal circumstances.
This information has been prepared by PNHSAA Inc., is copyright protected and is used with their permission.

