The evidence for the effectiveness of Soliris as a treatment for PNH
With over 10 years of clinical evidence now available, there can be no questions as to the efficacy of Soliris (eculizumab) in reducing the harmful effects of the PNH disease.
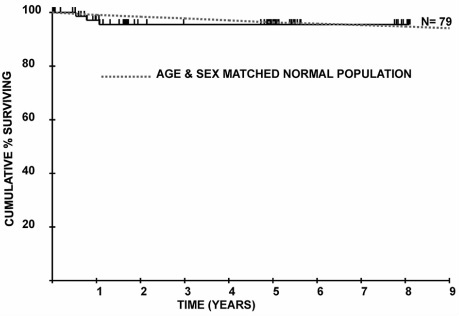
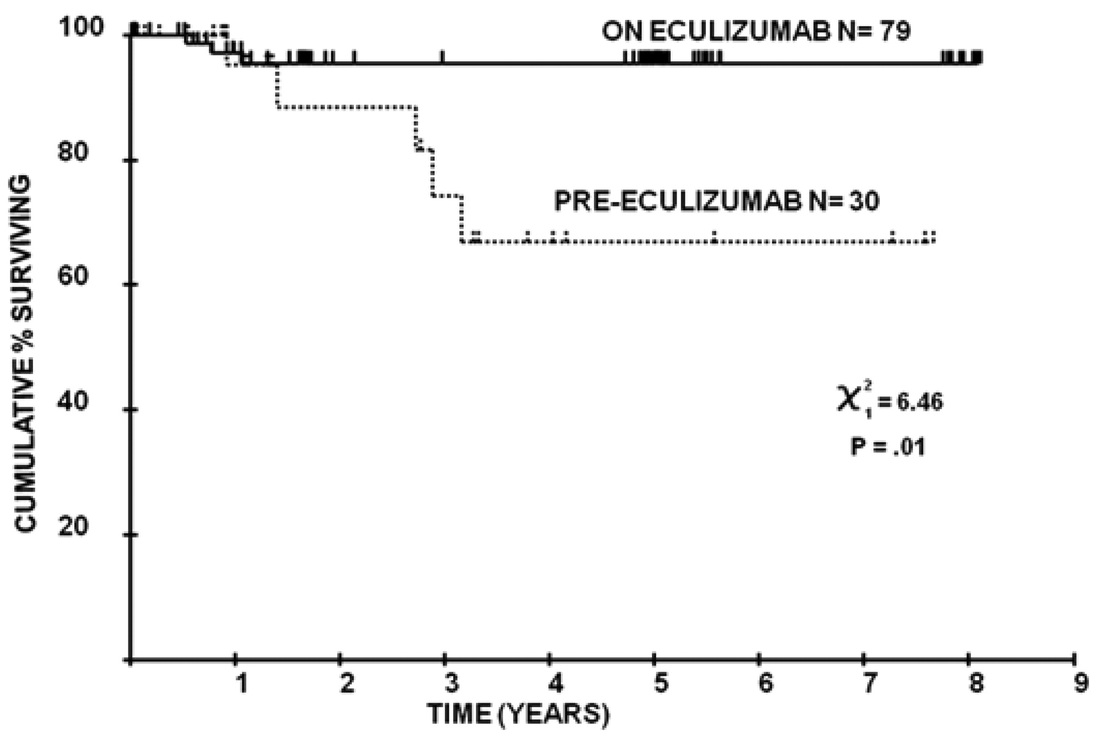
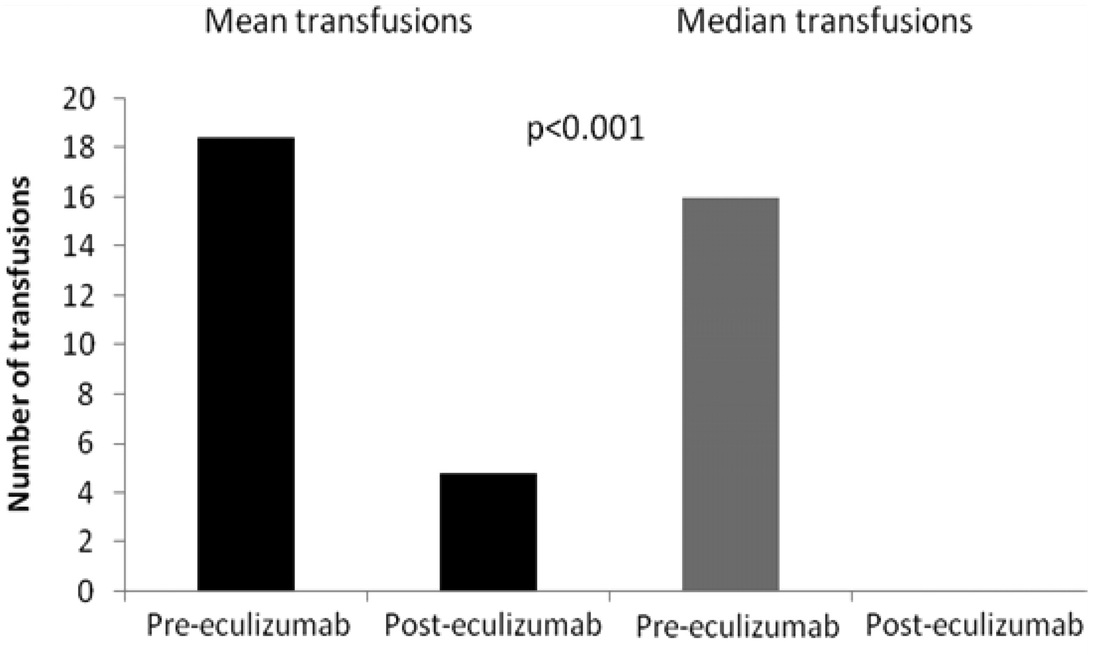
Research based on clinical experience in the United Kingdom, published in 2011, demonstrated that the Soliris treatment returned mortality rates for PNH patients to those of the general population. The findings from this research, "Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival," are shown below.
In 2012 further follow-up was conducted in the United Kingdom, with data now available from a 10 year period and outcomes for 153 patients. The findings from this report, "Eculizumab in Paroxysmal Nocturnal Hemoglobinuria (PNH): A Report of All 153 Patients Treated in the United Kingdom 10-Year Experience," are also shown below.
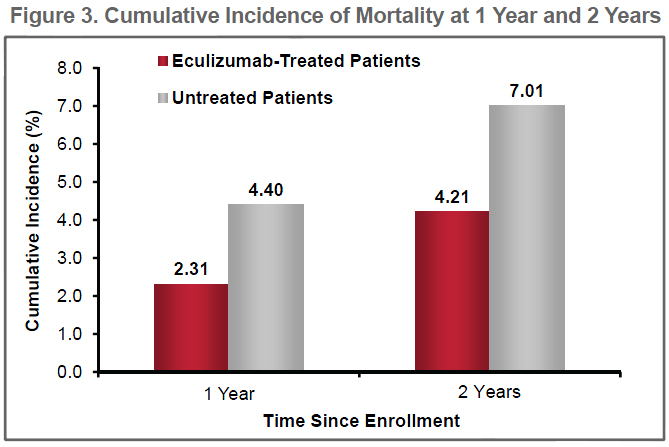
Further analysis is provided below in the report, "Eculizumab Protects Against Thromboembolism and Prolongs Survival in Patients with Paroxysmal Nocturnal Hemoglobinuria: An International PNH Registry Study". This data comes from the International PNH Registry, with 1547 PNH patients registered internationally.
Research based on clinical experience in the United Kingdom, published in 2011, demonstrated that the Soliris treatment returned mortality rates for PNH patients to those of the general population. The findings from this research, "Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival," are shown below.
In 2012 further follow-up was conducted in the United Kingdom, with data now available from a 10 year period and outcomes for 153 patients. The findings from this report, "Eculizumab in Paroxysmal Nocturnal Hemoglobinuria (PNH): A Report of All 153 Patients Treated in the United Kingdom 10-Year Experience," are also shown below.
Further analysis is provided below in the report, "Eculizumab Protects Against Thromboembolism and Prolongs Survival in Patients with Paroxysmal Nocturnal Hemoglobinuria: An International PNH Registry Study". This data comes from the International PNH Registry, with 1547 PNH patients registered internationally.
Definitions
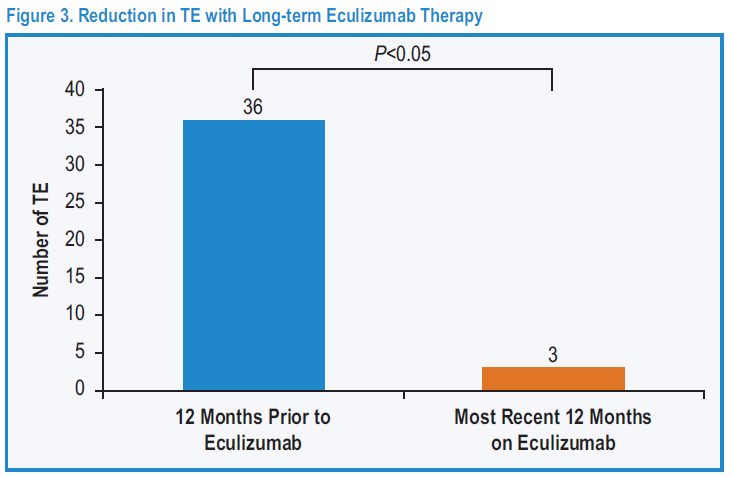
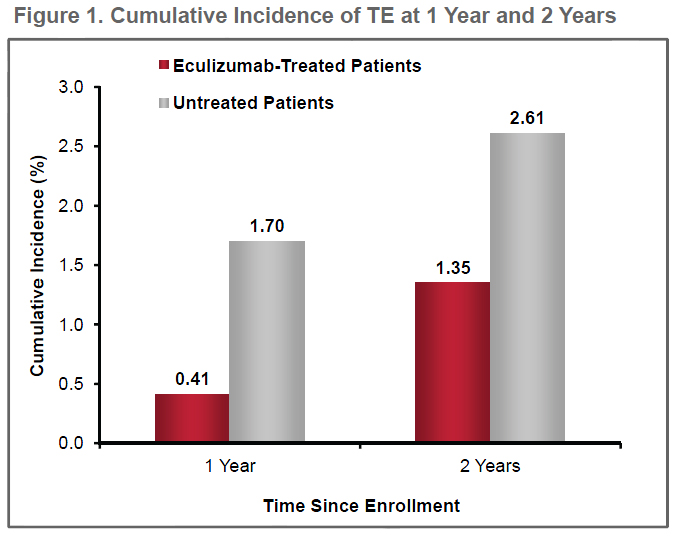
Thromboembolism (TE): Formation in a blood vessel of a clot (thrombus) that breaks loose and is carried by the blood stream to plug another vessel. The clot may plug a vessel in the lungs (pulmonary embolism), brain (stroke), gastrointestinal tract, kidneys, or leg. Thromboembolism is a significant cause of morbidity (disease) and mortality (death).
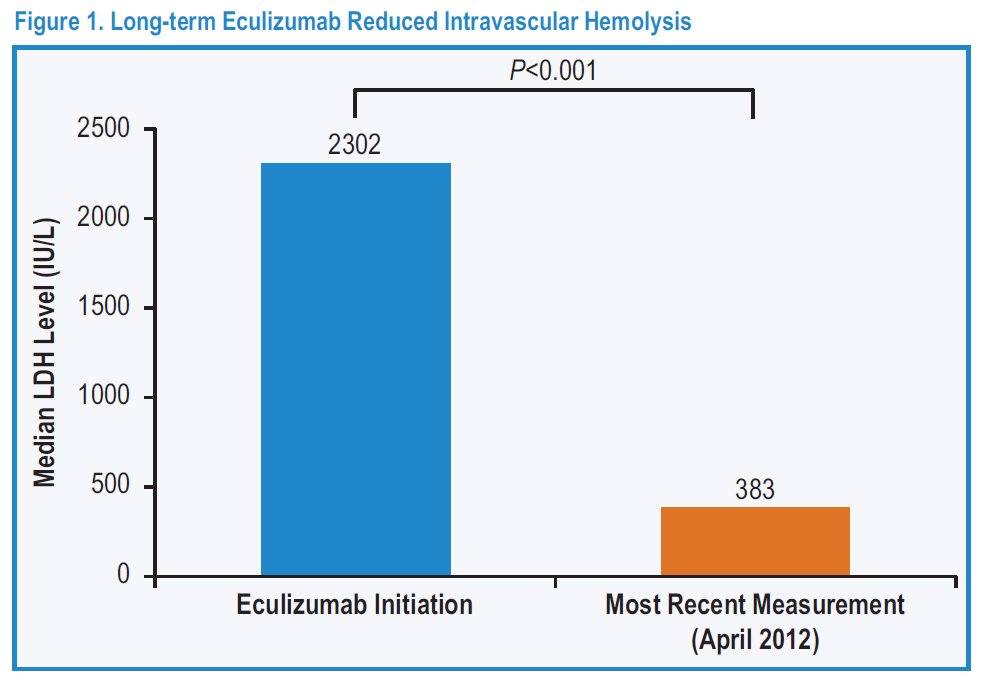
Intravascular Hemolysis: The destruction of red blood cells within the circulation, leading to the release of haemoglobin from within the red blood cells into the blood plasma.
Eculizumab in Paroxysmal Nocturnal Hemoglobinuria (PNH): A Report of All 153 Patients Treated in the United Kingdom 10-Year Experience.
Conclusions
Patient Characteristics (N = 153)
Authors: Anita Hill, MBChB, PhD, Richard J. Kelly, MBChB, Austin G. Kulasekararaj, MBBS, MD, Shreyans A. Gandhi, MBBS, MD, Lindsay D. Mitchell, MBChB, Modupe Elebute, MBChB, MD, Stephen John Richards, PhD, Matthew Cullen, MSc, Louise M. Arnold, RN, BSc, Joanna Large, RN, BSc, Alexandra Wood, RN, BSc, Gemma L. Brooksbank, RN, BSc, Tracy Downing, Claire McKinley, BSc, Dena Cohen, BSc, MSc, Walter M. Gregory, PhD, Judith C. W. Marsh, MD, PhD, Ghulam J. Mufti, MD, Peter Hillmen, MBChB, PhD Download |
Eculizumab Protects Against Thromboembolism and Prolongs Survival in Patients with Paroxysmal Nocturnal Hemoglobinuria: An International PNH Registry Study
Conclusions
Patient Characteristics (N=1047)
Authors: Gerard Socié, Hubert Schrezenmeier, Petra Muus, Jeff Szer, Alvaro Urbano-Ispizua, Jaroslaw P. Maciejewski, Robert Brodsky, Monica Bessler, Yuzuru Kanakura, Wendell Rosse, Gus Khursigara, Camille Bedrosian, Peter Hillmen Download |
Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survivalPatients and investigations
Seventy-nine patients with PNH were treated with eculizumab at Leeds Teaching Hospitals PNH center between May 2002 and July 2010, including 34 patients from the clinical trials and a further 45 treated since then. Funding was through the National Health Service either via central commissioning in England or local funding in Wales or Scotland. The follow-up of patients was approved through the Central Research Ethics Committee, and all patients gave signed informed consent for entry into the study in accordance with the Declaration of Helsinki. The diagnosis was established or confirmed using multicolor flow cytometry of the erythrocytes and granulocytes in all cases. Patients were assessed at a minimum of every 12 weeks by the Leeds PNH team. Data collected for analysis include age and symptoms at diagnosis, presence of a history of preceding aplastic anemia (AA) or myelodysplasia (MDS), age at the start of eculizumab, use of other medication to treat PNH, the occurrence of thrombosis before and during eculizumab treatment, blood transfusion requirement for the 12 months before starting eculizumab and after its initiation, lactate dehydrogenase (LDH) levels at the start of eculizumab and the most recent level (all of which were from May, June, or July 2010), and documentation of cause and date of death if applicable. Data were collected up until the end of July 2010 when this planned analysis was made. In the 7 years before the availability of eculizumab, 30 patients who fulfilled our criteria for eculizumab therapy were cared for in Leeds. The mortality of these patients, up to the point at which they went on to receive eculizumab, was evaluated to provide a control group for comparison. Authors: Anita Hill, Louise M. Arnold, Gemma L. Brooksbank, Stephen J. Richards, Matthew Cullen, Lindsay D. Mitchell, Dena R. Cohen, Walter M. Gregory, and Peter Hillmen Download |
Figure 1: Overall survival of 79 patients from initiation of eculizumab treatment compared with an age- and sex-matched normal population.
Figure 3: Overall survival of patients before and after eculizumab.
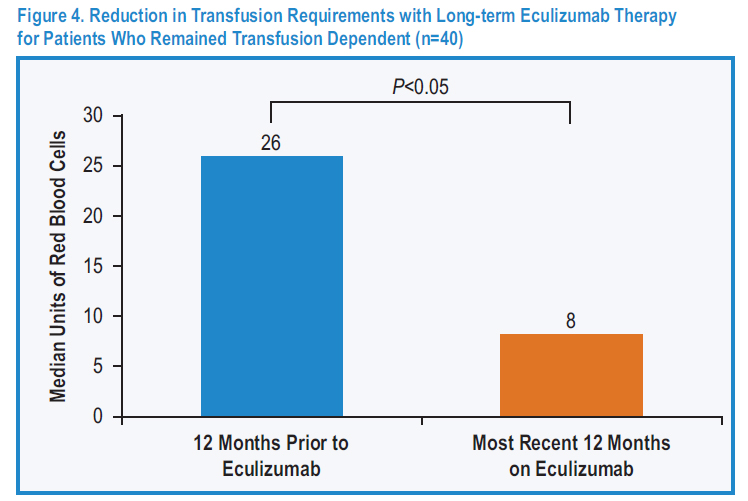
Figure 4: Blood transfusion requirements in the 12 months before eculizumab therapy and the most recent 12 months on eculizumab treatment in 64 patients.
|